-40%
Digital pupilometer PD meter Optical Optometry LCD Display Automatic Shut-off
$ 41.7
- Description
- Size Guide
Description
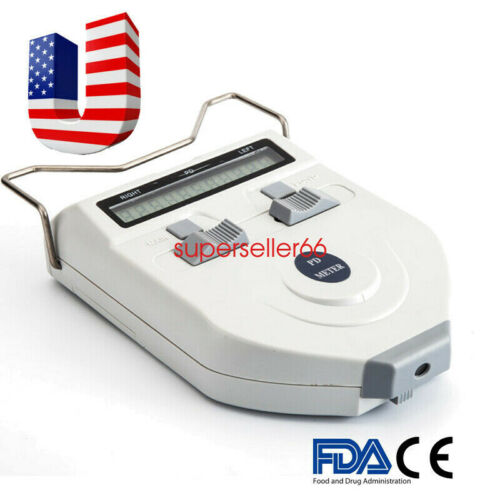
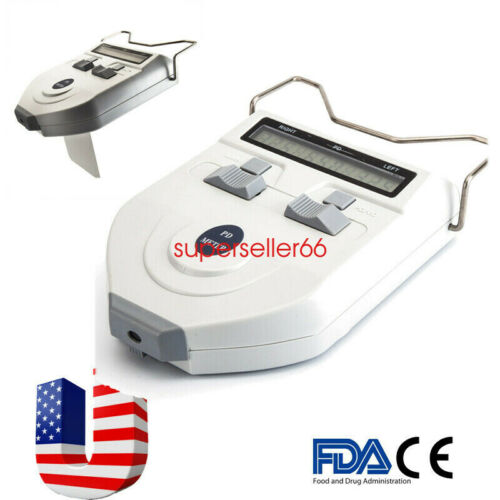
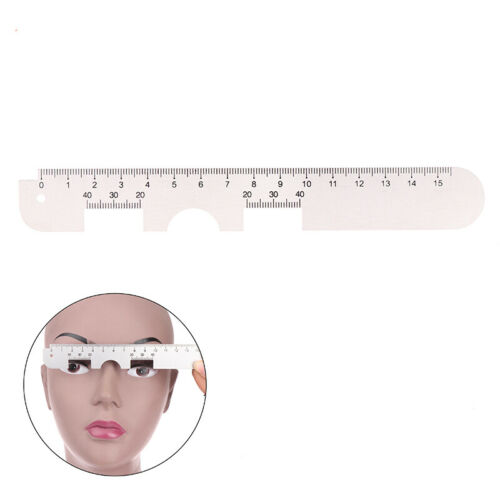
Description:Model: 310061
Features:
Using mechanical hairspring to level at the reflecting point of human cornea to realize the consecutive
measurement. It features direct point sampling and high accuracy of orientation;
Adopting high-precision line-shaped sensors, advanced intellectualized electronic system and digital
display
which allow the testing results more visible,legible and accurate;
The design of LED light source and low power consumption, long service life of the battery;
LED light source, time of auto power off could be adjustable. There is a +2.00D lens under measuring
window to make a visual compensation.
Specification:
Color:White
Range of measurement:45~82mm(total) in 0.5mm steps,22.5~41mm(each) in 0.5 steps
Step: 0.5mm
Time for Automatic Shut-off:
About 1 Minute After Stopping Operation, or Turn It off Manually
Power Supply:AA batteries(Not Include)
Dimensions:220x160x55mm
Net Weight: 0.7kg
Package Include
:
1 X Digital PD Meter
FDA Disclaimer:
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
ARTG NO: 278908 ARTG Number: 278908
Statement: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item. (The seller name: Helen Zhang,City:Beijing State: Beijing Country: China Telephone number: 86-18730611603)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013).
Payment Mathord
1. PayPal payment accepted only.
2. Items will be shipped to your eBay address. Please make sure it is correct.
3. Payment must be received 4 days after auction ended.
Returns Policy
1. Customer satisfaction is our top goal. We believe our items are so outstanding. All products are quality checked. They are new and in good condition when shipped to our customers. We are convinced you will be happy with your Purchase.
2. If product is defective or damage upon arrival, or wrong product shipped, please contact us immediately. Returns accepted within 14 days of delivery date and item must be in original new condition, not worn or altered in any way with attached tags & wrap. Otherwise deal is final. Return shipping must be paid by buyer.
3. Please contact us first if you have any problems/questions/concerns. We will be happy to resolve any issues you may have in a cordial and friendly manner.
4. We appreciate your Postive Feedback, and will do the same in return. DO NOT leave negative feedback without first communication with us. Please allow max 2 business days for us to response.
About Feedback
When you satisfied with our product and services please leave us positive feedback.
If a problem occurs, contact us immediately with any email request. We promise we will give you the satisfied solution. :)
Contact US
The seller’s name: Alice
City:Beijing
State: Beijing
Country: China
Telephone number: 86-18305265146
FDA Disclaimer:
Statement: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013). You can consult with the FDA's Center for Devices and Radiological Health